- A-29, Industrial Area, Site IV,
Sahibabad, Ghaziabad, UP, India. - (+91-120) 2896063
info@bhartiyagroups.com

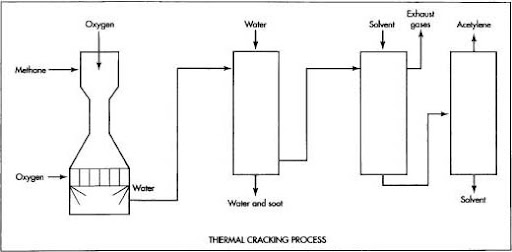
There are two ways to produce acetylene; you can use a chemical reaction process or you can use thermal cracking. In the chemical reaction process the ratio of hydrogen to carbon is 2:1 and in thermal cracking the same ratio is used but it is produced under high pressure and temperature twice that of the chemical reaction process.
The Manufacture of Acetylene (C 2 H 2): The manufacture of acetylene from calcium carbide is actually quite simple. In a reactor, the calcium carbide is placed in a suspension with water, hydrochloric acid and a small amount of sulfuric acid. The materials are stirred for approximately one hour, then heated to nearly 500oC. During this process the calcium carbide reacts with water to form calcium hydroxide and methane gas.
This is the manufacturing process of Acetylene. Acetylene (AC), also called acetic acid ethylene, C 2 H 2, is a colorless and odorless gas, the simplest alkyne in organic chemistry, with formula CH 3 -CO-CH 3. It is the simplest alkali metal hydride and combining form of acetylide anion. Under normal conditions as a gas, acetylene exists as two vibrational bands; just as visible light, which also has two vibrational bands that defines its color and perceived brightness.
The process begins by mixing Acetylene, Calcium Carbide and water in a reaction vessel via a pipeline. This reaction produces calcium sulfide, carbon dioxide and hydrogen gas. Next, the mixture is distilled to remove excess carbon dioxide, which is then recycled back into the process. The liquid phase of the mixture is then removed in order to obtain solid product of calcium sulfide. This material is then heated to form calcium sulfide into calcium carbide. The calcium carbide is then cooled and ground back down into fine grain powder form which is known as raw acetylene gas.
The process of thermal cracking used in manufacturing acetylene has two stages. First, acetylene is produced by heating calcium carbide CaC2 at high temperature. The CaC2 combines with water (H2O) to form Ca(OH)2 and C2H4. The initial stage is designed to separate the heavy hydrocarbon C5+ from the C4.
In the thermal cracking process, high-pressure steam is flashed into high-pressure (50–60 bar) liquid acetylene at 400–450 °C (750–850 °F). The resulting low-pressure vapors are passed over a catalytic cracker to achieve higher octane rating than normal crude oil distillate.
A relatively inexpensive and highly reactive chemical, acetylene is processed by various companies in different ways. In South Africa, the most common method is thermal cracking, where the acetylene, which is a gas at normal temperature and pressure, needs to be changed into a liquid before it can be further processed.
Bhartiya Cryogas is top manufacturer of hi tech acetylene plants India. It’s fully automatic Acetylene plants are available in capacities from 25m3 (cubic meter) /hr to 200m3/Hr. In a Stationary Carbide to Water type Automatic Acetylene Generator acetylene is produced by reaction of calcium carbide with water. Adequate quantity of water is held in the generator shell to which calcium carbide is fed from top. The following reaction takes place:- CaC2+2H2O = C2H2 + Ca(OH)2 + 27,000 Calories (heat generated).
The design of the entire plant has been made with safety in mind. Flash Back Arrestors, High Pressure Reverse Flow check valves, safety valves and complete gauging help protect both equipment and personnel. Various auto controls, safety valves, auto vent valves, non-return valves and Flash Back Arrestors are also provided to make this plant absolutely safe to operate in all conditions.
Acetylene Plant, Acetylene plant India, Acetylene plants, BhartiyaCryogas, Calcium Carbide, Chemical Reaction Process, Thermal Cracking







